Sour Taste: Chemical or Physical? Mind-Blowing Science!

Sour taste, a fundamental aspect of gustation, often prompts scientific inquiry. Understanding the mechanism behind sourness requires investigating its relationship to acids, particularly concerning whether the sensation is sour taste a chemical or physical property. The role of hydrogen ions (H+) in stimulating taste receptor cells is central to this investigation, potentially offering clarity. Researchers at institutions like the Monell Chemical Senses Center actively investigate these taste receptor mechanisms, contributing to our knowledge about the underlying principles governing sour taste perception.
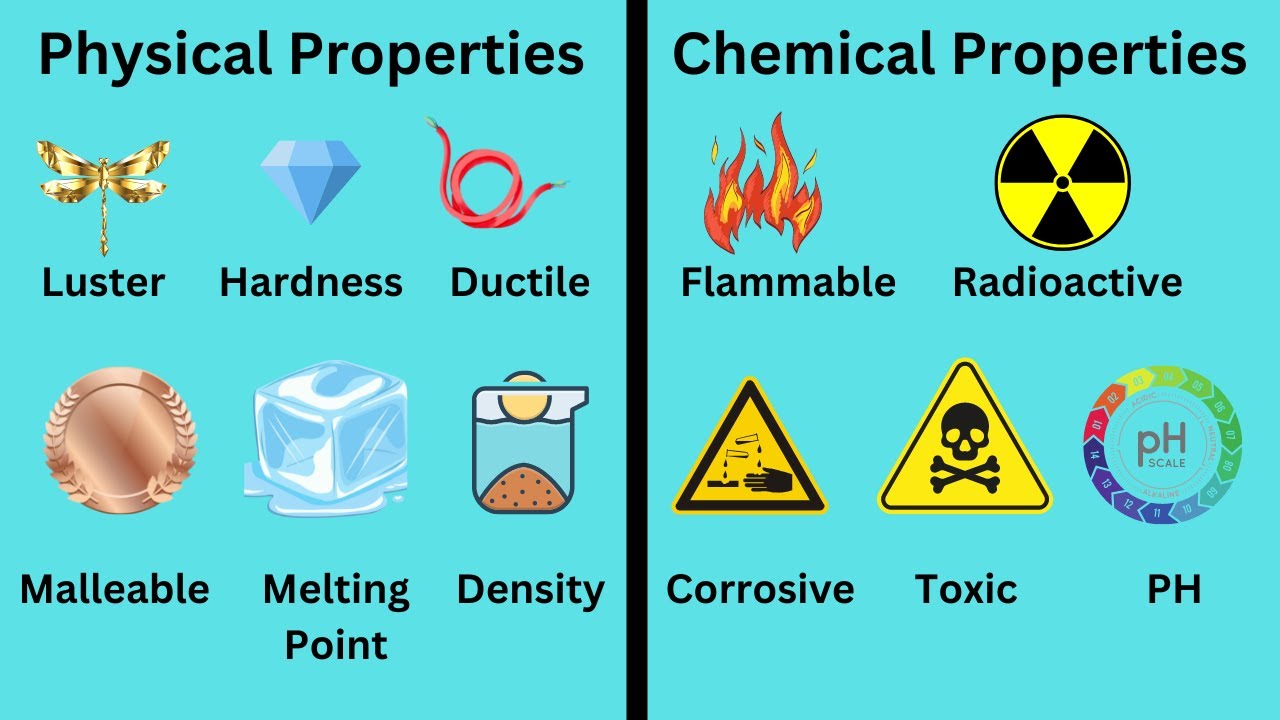
Image taken from the YouTube channel MooMooMath and Science , from the video titled Physical and Chemical Properties .
Identifying Key Players: The Entities Involved in Sour Taste Perception
Before we can delve into whether sour taste is a chemical or physical property, it's crucial to establish a shared understanding of the elements at play. This involves identifying and defining the various entities involved in the perception of sour taste, setting the stage for a deeper exploration of their interactions. This foundational knowledge will allow us to dissect the complex mechanisms that ultimately lead to our sensory experience of "sourness."
The Foundation of Sour Taste Perception
At its core, sour taste is a sensory experience. It’s the perception we have when specialized receptors in our mouth are stimulated by acids.
But this seemingly simple sensation is far from a solo act. It involves a cast of characters, each with a specific role in the overall process.
Let’s meet the key players:
The Elicitors: Acids
Acids are the primary substances responsible for eliciting sour taste. Common examples include:
-
Citric acid: Found in citrus fruits like lemons and limes.
-
Lactic acid: Present in fermented foods like yogurt and sauerkraut.
-
Acetic acid: The main component of vinegar.
Acids are characterized by their ability to donate hydrogen ions (H+) in solution, and it is these ions that ultimately trigger the sour taste receptors.
The Receptors: Gatekeepers of Sourness
Embedded within our taste buds are specialized taste receptors specifically sensitive to sour stimuli. These receptors are proteins designed to bind with hydrogen ions.
While the precise mechanisms are still being researched, the OTOP1 ion channel protein is a known key player. These receptors act like gatekeepers, initiating a chain of events when they encounter an acid.
The Stage: Tongue and Saliva
The tongue is the primary organ responsible for taste perception. Its surface is covered in taste buds, which house the taste receptor cells.
Saliva plays a crucial role as well. This watery fluid dissolves the food substances, allowing them to interact with the taste receptors.

Without saliva, we wouldn’t be able to taste anything at all. It effectively acts as a solvent, facilitating the interaction between acids and receptors.
The Measure: pH
pH is a measure of acidity or alkalinity. It’s related to the concentration of hydrogen ions in a solution.
A lower pH indicates a higher concentration of hydrogen ions and therefore greater acidity. While not directly tasted, pH quantifies the strength of an acid.
The Science Behind It: Key Properties & Disciplines
To understand sour taste, it’s also vital to grasp the difference between chemical and physical properties.
A chemical property relates to a substance's ability to undergo a chemical change, such as reacting with another substance.
A physical property, on the other hand, is a characteristic that can be observed or measured without changing the substance’s chemical identity (e.g., boiling point, color).
Furthermore, several scientific disciplines converge to explain sour taste:
-
Taste perception is the broad process of sensing and interpreting tastes.
-
Sensory science is the overarching study of the senses, including taste, smell, and touch.
-
Gustation specifically refers to the sense of taste.
-
Food science explores all aspects of food, from production to consumption, including its sensory properties.
The Triggers and Messengers: Ions and Neurotransmitters
The magic behind sour taste lies within hydrogen ions (H+). These are the specific ions released by acids that directly trigger the sour taste receptors.
Ion channels, protein structures within cell membranes, then allow ions to flow across the cell membrane upon receptor activation.
This ion flow is essential for generating an electrical signal.
Neurotransmitters, chemical messengers released by the taste receptor cells, then transmit the signal to nerve cells.
These chemicals are the language the taste cells use to communicate with the brain.
The Interpreter: The Brain
Ultimately, it is the brain that interprets these signals and registers the sensation of "sour." The brain processes the incoming information from the taste receptors and creates our conscious perception of taste.
The Pathway: Signal Transduction
Signal transduction describes the entire process by which a signal is converted into a cellular response. In the context of sour taste, it includes:
- The binding of hydrogen ions to receptors.
- The opening of ion channels.
- The release of neurotransmitters.
- Signal transmission to the brain.
The Influencers: Smell and Temperature
Finally, it’s important to remember that taste doesn’t exist in isolation.
Olfaction (smell) significantly influences overall flavor perception. What we perceive as "taste" is often a combination of taste and smell.
Even temperature can affect chemical reactions and, therefore, taste perception.
Understanding these key players and their roles is essential for unraveling the nature of sour taste and determining whether it is primarily a chemical or physical property.
Before we dive deeper, it's important to understand how the initial interaction between acids and our taste receptors unfolds. This interaction is not merely a physical touch, but a cascade of chemical events that kickstarts the entire sour taste experience.
The Chemistry of Sour: How Acids Interact with Taste Receptors
The sourness we perceive isn't just a matter of taste buds meeting a substance. It's a carefully orchestrated chemical dance between acids and specialized receptors on our tongues. Understanding the nature of this interaction is key to grasping why sour taste is classified as a chemical property.
The Dissociation of Acids and Release of Hydrogen Ions
Acids, the primary elicitors of sour taste, don't exert their influence directly. Instead, they act as hydrogen ion (H+) donors. When an acid, such as citric acid in lemon juice or acetic acid in vinegar, is introduced into a water-based solution like saliva, it undergoes a process called dissociation.
This dissociation involves the acid molecule breaking apart, releasing H+ ions into the surrounding solution. The concentration of these H+ ions is what determines the acidity of the solution, measured by pH. The lower the pH, the higher the concentration of H+ ions, and generally, the more sour the substance will taste.
It's crucial to note that this release of H+ ions is a fundamental chemical reaction. It alters the composition of the acid, transforming it into its dissociated form and liberating the hydrogen ions that will go on to interact with our taste receptors.
The Interaction of H+ Ions with Taste Receptors
Once released, these H+ ions don't simply float around aimlessly. They seek out and interact with specialized taste receptors located on the surface of taste cells within our taste buds.
Research has identified specific proteins that function as sour taste receptors, most notably the OTOP1 ion channel. These receptors are strategically designed to bind with H+ ions.
The binding of H+ ions to the OTOP1 receptor is not a random event. It is a highly specific chemical interaction driven by the molecular shapes and electrical charges of the interacting molecules. The receptor protein has a binding site that complements the shape and charge of the H+ ion, allowing for a strong and selective association.
The Chemical Nature of Binding
The interaction between H+ ions and taste receptor proteins exemplifies a chemical reaction at the molecular level.
It involves the formation of chemical bonds, specifically electrostatic interactions, between the positively charged H+ ions and negatively charged regions within the receptor protein's binding site.
This binding event triggers a conformational change in the receptor protein, altering its shape and initiating a cascade of downstream events that ultimately lead to the perception of sourness.
The specificity of this binding is paramount. Only molecules with the correct shape and charge distribution, like H+ ions, can effectively bind to the receptor and elicit the sour taste response. This underscores the chemical specificity of the sour taste mechanism.
Temperature's Influence on the Reaction
While the interaction between acids and taste receptors is inherently chemical, temperature can play a modulatory role. Temperature affects the rate of chemical reactions.
Higher temperatures generally increase the rate of molecular motion and collision, potentially accelerating the dissociation of acids and the binding of H+ ions to receptors.
However, extremely high temperatures can also denature proteins, including taste receptors, disrupting their structure and function. Similarly, very low temperatures can slow down the reaction rates.
The influence of temperature on sour taste perception is complex. While temperature itself doesn't cause sourness (acids do), it can subtly alter the intensity of the perceived sourness by influencing the underlying chemical reaction rates.
The release of H+ ions sets the stage, but it's only the first act in a much larger performance. Once those ions bind to the taste receptors, a flurry of activity ensues, transforming a simple chemical interaction into the complex sensation we know as sourness.
Signal Transduction: From Receptor to Brain
The journey from the tongue to the brain is a fascinating example of biological signal transduction. The initial binding of hydrogen ions to taste receptors is merely the trigger that sets off a cascade of events. These events amplify and transmit the signal, ultimately leading to the perception of sour taste.
Opening the Floodgates: Ion Channel Activation
The sour taste receptors, such as the OTOP1 channels, are specialized proteins embedded in the cell membrane of taste receptor cells. These channels are normally closed, preventing the flow of ions across the membrane.
However, when H+ ions bind to these receptors, a conformational change occurs. This change causes the ion channel to open, creating a pathway for ions to flow into and out of the cell.
This opening is a crucial step, as it converts the chemical signal (H+ binding) into an electrical signal that the nervous system can understand.
The Ionic Current: Flow Across the Cell Membrane
With the ion channels now open, ions rush across the cell membrane, driven by electrochemical gradients. These gradients are established by differences in ion concentrations and electrical potential between the inside and outside of the cell.
The specific ions that flow through the channels vary depending on the type of taste receptor cell. However, the influx of positively charged ions (such as sodium or calcium) generally leads to a depolarization of the cell membrane.
This depolarization is a change in the cell's electrical potential, making the inside of the cell more positive relative to the outside.
This change in electrical potential is a key signal that triggers further events in the signal transduction pathway.
Neurotransmitter Release: Sending the Message
The depolarization of the taste receptor cell eventually reaches the cell's terminals, where it triggers the release of neurotransmitters.
Neurotransmitters are chemical messengers that transmit signals between nerve cells. In the case of sour taste, the release of neurotransmitters from the taste receptor cell is the mechanism by which the "sour" signal is passed on to the next neuron in the pathway.
The specific neurotransmitters involved in sour taste perception are still being investigated, but they likely include a combination of different signaling molecules.
To the Brain: Transmission of the Sour Signal
Once released, neurotransmitters diffuse across the synapse (the gap between nerve cells) and bind to receptors on the postsynaptic neuron.
This binding triggers a new electrical signal in the postsynaptic neuron, which then propagates along the neuron's axon towards the brain.
This process is repeated at each synapse along the pathway, allowing the "sour" signal to be transmitted from the tongue, up through the cranial nerves, and ultimately to the brainstem and gustatory cortex.
Interpretation: The Brain's Perception of Sour
The final stage of signal transduction occurs in the brain, where the incoming signals are interpreted as "sour." The gustatory cortex, a specialized region of the brain responsible for processing taste information, receives input from various taste receptor cells and integrates this information to create a unified taste perception.
The intensity of the sour taste is likely encoded by the frequency of action potentials (electrical signals) firing in the taste neurons. A higher frequency of firing generally indicates a stronger stimulus.
Additionally, the brain integrates information from other senses, such as smell and texture, to create the overall flavor experience. This integration explains why our perception of sourness can be influenced by factors such as the aroma of the food or the temperature of the beverage.
Ions, driven by electrochemical gradients, flood across the cell membrane, creating an ionic current. This current depolarizes the taste receptor cell, triggering a cascade of events that ultimately leads to the release of neurotransmitters. But why is this intricate process considered a chemical property, and not simply a physical one? The answer lies in understanding the fundamental differences between chemical reactions and physical changes.
Why Not Physical? Distinguishing Chemical Reactions from Physical Changes
The perception of sourness is not merely a matter of physics. While physical factors certainly influence our overall sensory experience, the core mechanism behind sour taste is rooted in chemical interactions. Let's explore why sourness transcends simple physical alteration.
Understanding Physical Changes
A physical change alters the form or appearance of a substance, but not its chemical composition. For example, melting ice is a physical change because it transforms water from a solid to a liquid, but it remains H2O.
Similarly, dissolving sugar in water is a physical change; the sugar molecules disperse throughout the water, but they are still sugar molecules.
The chemical identity of the substance remains unchanged.
The Chemical Basis of Sourness
In contrast, sour taste arises from a chemical reaction between acids and taste receptors. Acids, when dissolved in saliva, release hydrogen ions (H+).
These H+ ions then bind to specific receptor proteins on taste receptor cells, such as OTOP1 channels.
This binding is not a simple physical association; it's a chemical reaction that alters the receptor protein's conformation, initiating a cascade of events.
Temperature: A Modulator, Not a Cause
Temperature can influence our perception of taste. For example, warmer temperatures can enhance the perceived sweetness of a substance.
However, temperature doesn't inherently cause sourness itself. A substance isn't sour because it's hot or cold.
Sourness requires the presence of an acid and its chemical interaction with taste receptors.
While temperature can modulate the intensity of the sour sensation by affecting the rate of chemical reactions, it doesn't instigate the fundamental sourness the way an acid does.
Chemical Reaction vs. Physical Perception
Consider the difference: Cooling a lemon will not remove its sourness. The citric acid remains present, and its potential to release H+ ions and interact with taste receptors persists.
Conversely, neutralizing the citric acid with a base will eliminate the sourness. This is because the chemical reaction responsible for generating the sour taste has been prevented.
In summary, the chemical reaction between acids and taste receptors is the defining characteristic of sour taste. While physical factors such as temperature can influence the perception of sourness, they do not cause it. The underlying mechanism remains fundamentally chemical.
Video: Sour Taste: Chemical or Physical? Mind-Blowing Science!
FAQs: Sour Taste - Chemical vs. Physical
Still puzzling over whether that lemon's sourness is chemistry or physics? Here are some quick answers to common questions.
What exactly determines if something tastes sour?
Sourness is determined by the concentration of hydrogen ions (H+) released by acids in the food or drink. These ions trigger specific receptors on our tongue, which send signals to the brain, resulting in the sour taste sensation. Therefore, sour taste is a chemical property.
Is sour taste a chemical or physical property, and why is it important to know the difference?
Sour taste is a chemical property because it relates to a substance's ability to undergo a chemical reaction—in this case, releasing H+ ions in the presence of saliva. Understanding the difference helps in classifying and predicting how substances will interact with our bodies and the environment.
Can something be sour without actually being acidic?
While sourness is primarily linked to acidity (the presence of H+ ions), the intensity of sourness can be influenced by other factors. Temperature, other tastes present (like sweetness), and individual sensitivity can modify how strongly we perceive sourness. However, fundamentally, to be sour, the substance must have the chemical capability to release H+ ions.
How does our sense of taste differentiate between sour and other flavors like salty or sweet?
Our taste buds contain specialized receptor cells for each of the basic tastes (sour, salty, sweet, bitter, umami). These receptors are activated by different chemical compounds. In the case of sour, it's the hydrogen ions binding to specific receptors, leading to a distinct neural signal that our brain interprets as "sour". Thus, the difference is based on which specific chemical reaction triggers which receptor.
