Proton & Neutron Mass: A Simple Guide in AMU [Explained]

The fundamental particles within the atom, protons and neutrons, each possess a specific mass. Expressed in atomic mass units (AMU), this mass is crucial for understanding nuclear physics. The Dalton, another name for the AMU, provides a standardized unit for measuring atomic and molecular weights. Researchers at the National Institute of Standards and Technology (NIST) constantly refine the measurement of these fundamental constants. Understanding the mass of proton and neutron in amu is key for anyone studying chemistry or physics, including the work of scientists like Ernest Rutherford, who pioneered the field of nuclear physics.
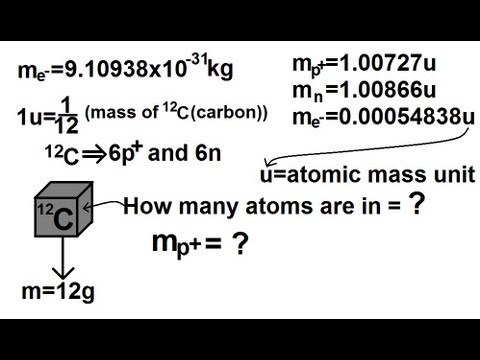
Image taken from the YouTube channel Michel van Biezen , from the video titled Physics - Nuclear Physics (1 of 22) Mass of Proton, Neutron, and Electron .
Understanding Proton & Neutron Mass in Atomic Mass Units (AMU)
This guide aims to provide a clear and straightforward explanation of the mass of protons and neutrons, specifically when expressed in atomic mass units (AMU). We will explore what AMU represents and why it's a useful unit for describing the mass of these subatomic particles.
What is the Atomic Mass Unit (AMU)?
Defining AMU: A Standard Unit
The atomic mass unit (AMU), often denoted as 'u' or 'Da' (Dalton), is a unit of mass used to express the masses of atoms and molecules. It's defined as 1/12 the mass of a neutral carbon-12 (12C) atom in its ground state. This provides a convenient reference point for measuring incredibly small masses.
Why Use AMU Instead of Grams or Kilograms?
Using grams or kilograms to express the mass of protons and neutrons results in extremely small numbers, making them cumbersome to work with in calculations and discussions. AMU provides a more manageable and relatable scale when dealing with the atomic and subatomic world. Imagine measuring the distance between cities in millimeters - kilometers makes much more sense!
Mass of Proton and Neutron in AMU
Proton Mass in AMU
The mass of a proton is approximately 1.007276 AMU. This value is determined experimentally using mass spectrometry and other precise measurement techniques. For most practical purposes, especially in introductory chemistry and physics, the proton mass is often rounded to 1 AMU.
- Symbol: p or p+
- Charge: +1 elementary charge (positive charge)
- Approximate Mass: 1 AMU (1.007276 AMU more precisely)
Neutron Mass in AMU
The neutron, unlike the proton, has no electric charge. Its mass is slightly greater than that of a proton, measuring approximately 1.008665 AMU. Similar to the proton, the neutron mass can be approximated to 1 AMU for simplified calculations.
- Symbol: n or n0
- Charge: 0 (neutral charge)
- Approximate Mass: 1 AMU (1.008665 AMU more precisely)
Comparing Proton and Neutron Mass
While both protons and neutrons have masses close to 1 AMU, it's important to acknowledge the subtle difference. Neutrons are slightly heavier than protons. This difference, though small, is significant in nuclear physics and affects the stability of atomic nuclei.
| Particle | Approximate Mass (AMU) | Precise Mass (AMU) | Charge |
|---|---|---|---|
| Proton | 1 | 1.007276 | +1 |
| Neutron | 1 | 1.008665 | 0 |
Significance of Knowing Proton and Neutron Mass
Calculating Atomic Mass
The mass number of an atom (the total number of protons and neutrons in the nucleus) gives a close approximation of the atom's mass in AMU. This is because the mass of electrons is significantly smaller and usually negligible in these calculations.
Understanding Isotopes
Isotopes of an element have the same number of protons but different numbers of neutrons. Knowing the mass of a neutron in AMU helps in calculating the mass of different isotopes and understanding their properties.

Nuclear Reactions and Mass-Energy Equivalence
In nuclear reactions, such as nuclear fission and fusion, there is a slight change in mass. This mass difference is converted into energy, as described by Einstein's famous equation E=mc2. Accurate knowledge of proton and neutron masses is crucial for calculating the energy released in these reactions.
Video: Proton & Neutron Mass: A Simple Guide in AMU [Explained]
Frequently Asked Questions: Proton & Neutron Mass in AMU
Hopefully, this guide provided a clear explanation of atomic mass units and the mass of protons and neutrons. Here are some frequently asked questions to further clarify the topic.
Why is the mass of a proton and neutron in amu not exactly 1?
While often approximated as 1 amu, the actual mass of a proton is slightly greater than 1 amu (approximately 1.007 amu), and the mass of a neutron is also slightly greater than 1 amu (approximately 1.009 amu). These slight differences arise due to the complexities of their internal structure and binding energies.
Is the atomic mass unit (amu) the same as Dalton (Da)?
Yes, the atomic mass unit (amu) and the Dalton (Da) are equivalent units of mass. Both are defined as 1/12 of the mass of a carbon-12 atom in its ground state. Therefore, you can use them interchangeably when discussing the mass of proton and neutron in amu.
Why is it important to know the mass of proton and neutron in amu?
Understanding the mass of proton and neutron in amu is fundamental in chemistry and physics. It allows us to calculate the atomic mass of elements and molecules, which is crucial for stoichiometric calculations, understanding chemical reactions, and analyzing isotopic composition.
Does the mass of an electron significantly affect atomic mass calculations?
No, the mass of an electron is significantly smaller compared to the mass of proton and neutron in amu (approximately 1/1836 amu). Therefore, the electron's mass is often negligible in basic atomic mass calculations. However, it becomes important in very precise measurements.
