Unlock Water's Secrets: Ion Product Constant Explained!

The self-ionization of water, a fundamental principle in chemistry, dictates the equilibrium between H+ and OH- ions. Understanding Arrhenius acids and bases, pivotal concepts in solution chemistry, provides a framework for examining aqueous solutions. The pH scale, developed by Søren Peder Lauritz Sørensen, offers a practical method for quantifying the acidity or alkalinity of a solution. Determining what is the ion product constant for water, often denoted as Kw, is crucial for calculating the concentration of these ions and predicting solution behavior. Kw, a temperature-dependent value, represents the product of [H+] and [OH-] and offers critical insights into the dissociation equilibrium of water.

Image taken from the YouTube channel chemistNATE , from the video titled What is Kw (The Ion Product Constant of Water) .
Unlock Water's Secrets: The Ion Product Constant Explained
Understanding the behavior of water is crucial in chemistry. One of the key properties that governs its behavior is the ion product constant, which reveals insights into water's autoionization and acidity. We will explore this constant and its significance.
What is the Ion Product Constant for Water (Kw)?
The ion product constant for water, often denoted as Kw, is the equilibrium constant for the autoionization of water. This seemingly simple concept explains how water molecules spontaneously react with each other to form hydronium ions (H₃O⁺) and hydroxide ions (OH⁻).
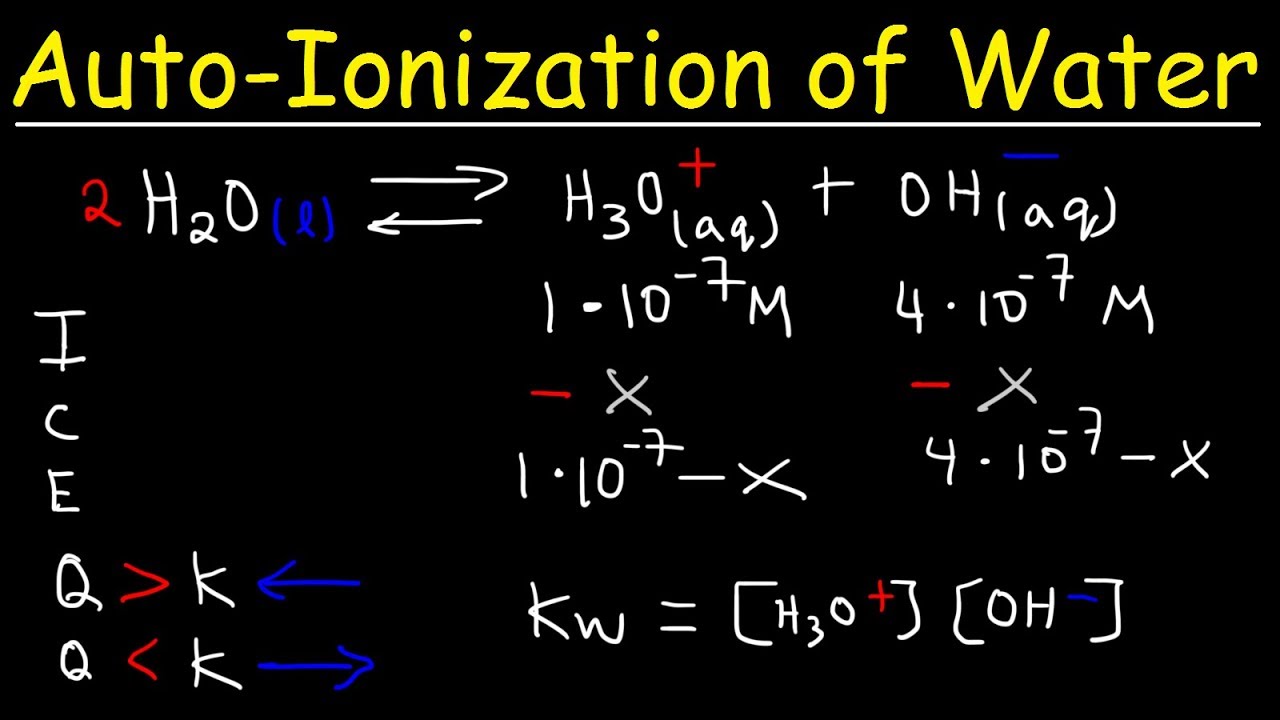
The Autoionization of Water
Pure water isn't just H₂O molecules. A tiny fraction of water molecules undergo autoionization, meaning they act as both an acid and a base with each other. The simplified chemical equation illustrating this process is:
H₂O(l) + H₂O(l) ⇌ H₃O⁺(aq) + OH⁻(aq)
It's important to note:
- This reaction is an equilibrium. There is a constant exchange happening between reactants and products.
- The equilibrium heavily favors the reactants (H₂O). Only a very small number of water molecules are ionized at any given time.
Defining the Ion Product Constant (Kw)
The ion product constant (Kw) mathematically expresses the extent of this autoionization. It is defined as the product of the hydronium ion concentration ([H₃O⁺]) and the hydroxide ion concentration ([OH⁻]) at a given temperature:
Kw = [H₃O⁺] [OH⁻]
Temperature Dependence of Kw
Kw is not a fixed value; it changes with temperature.
- Endothermic Process: The autoionization of water is an endothermic process, meaning it absorbs heat.
- Temperature Increase: As temperature increases, the equilibrium shifts to favor the formation of H₃O⁺ and OH⁻ ions. This results in a larger Kw value.
- Temperature Decrease: Conversely, as temperature decreases, the equilibrium shifts to favor the formation of H₂O, resulting in a smaller Kw value.
The following table illustrates how Kw changes with temperature:
| Temperature (°C) | Kw (approximate) |
|---|---|
| 0 | 0.11 x 10⁻¹⁴ |
| 25 | 1.0 x 10⁻¹⁴ |
| 50 | 5.47 x 10⁻¹⁴ |
| 100 | 49 x 10⁻¹⁴ |
The Significance of Kw
Kw provides a framework for understanding acidity and basicity in aqueous solutions.

Neutrality
A neutral solution is defined as one in which the concentration of hydronium ions equals the concentration of hydroxide ions: [H₃O⁺] = [OH⁻]. At 25°C, where Kw = 1.0 x 10⁻¹⁴, a neutral solution has:
[H₃O⁺] = [OH⁻] = 1.0 x 10⁻⁷ M
Acidity and Basicity
Kw allows us to determine whether a solution is acidic or basic:
- Acidic Solution: In an acidic solution, [H₃O⁺] > [OH⁻]. Since Kw = [H₃O⁺] [OH⁻] remains constant at a given temperature, an increase in [H₃O⁺] results in a decrease in [OH⁻].
- Basic Solution: In a basic solution, [OH⁻] > [H₃O⁺]. Similarly, an increase in [OH⁻] causes a decrease in [H₃O⁺] to maintain the Kw value.
Calculating pH and pOH
The concepts of pH and pOH are directly related to Kw.
- pH: pH is defined as the negative logarithm (base 10) of the hydronium ion concentration: pH = -log₁₀[H₃O⁺].
- pOH: pOH is defined as the negative logarithm (base 10) of the hydroxide ion concentration: pOH = -log₁₀[OH⁻].
The relationship between pH, pOH, and Kw is expressed as:
pH + pOH = pKw
Where pKw = -log₁₀Kw. At 25°C, pKw = 14.
This relationship allows us to easily calculate either pH or pOH if we know the other value, or the concentration of either hydronium or hydroxide ions.
Video: Unlock Water's Secrets: Ion Product Constant Explained!
FAQs: Understanding the Ion Product Constant of Water
These frequently asked questions will help you grasp the key concepts behind the ion product constant of water.
What exactly does the ion product constant (Kw) represent?
The ion product constant (Kw) represents the equilibrium constant for the self-ionization of water. It quantifies the extent to which water molecules dissociate into hydrogen ions (H+) and hydroxide ions (OH-) at a given temperature. It essentially tells us how much pure water breaks down into its constituent ions.
How does temperature affect the ion product constant for water?
Temperature has a significant impact on Kw. As temperature increases, the self-ionization of water increases, leading to a higher concentration of H+ and OH- ions. Consequently, the ion product constant for water also increases with rising temperatures. This is because the reaction is endothermic.
Why is Kw considered a constant, and what is the ion product constant for water at 25°C?
Kw is considered a constant because, at a specific temperature, the product of the concentrations of H+ and OH- ions in water remains constant, regardless of the presence of acids or bases. At 25°C, the ion product constant for water is approximately 1.0 x 10^-14. This means that [H+][OH-] = 1.0 x 10^-14 at this temperature.
How is the ion product constant used to determine the acidity or alkalinity of a solution?
Kw is crucial for determining the acidity or alkalinity of a solution because it establishes the relationship between [H+] and [OH-]. If [H+] > [OH-], the solution is acidic. If [H+] < [OH-], it's alkaline. Knowing Kw allows us to calculate one concentration if the other is known, helping determine whether a solution is acidic, basic, or neutral.