Carbohydrates: The Chemistry You Need to Know (Explained)

Understanding what is the chemical composition of carbohydrates is fundamental to grasping energy metabolism within biological systems. Biochemistry elucidates that carbohydrates, essential nutrients, are built from carbon, hydrogen, and oxygen atoms, typically in a ratio of 1:2:1. Glycolysis, a metabolic pathway, directly depends on the breakdown of these carbohydrates. The structures of these molecules vary from simple monosaccharides like glucose to complex polysaccharides such as starch, vital for energy storage in plants.
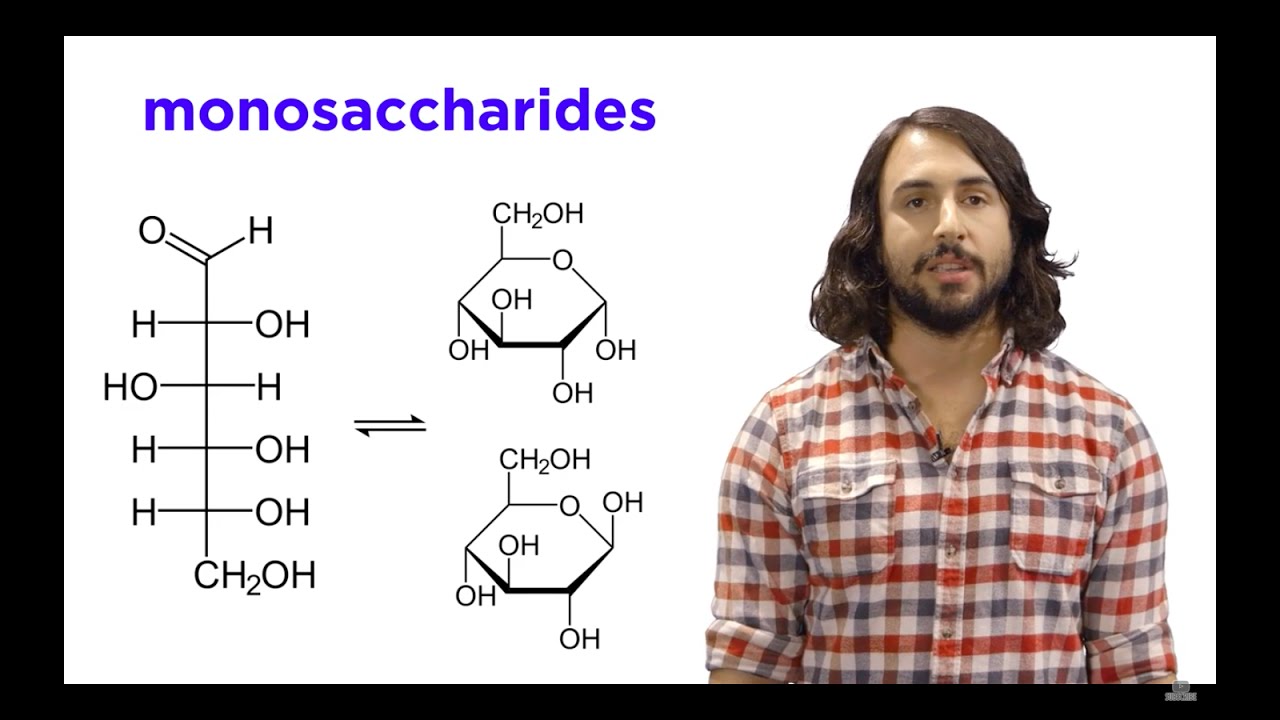
Image taken from the YouTube channel Professor Dave Explains , from the video titled Carbohydrates Part 1: Simple Sugars and Fischer Projections .
Deconstructing Carbohydrates: From Diet to Chemical Composition
Understanding carbohydrates involves more than just knowing they're in bread and pasta. It requires a grasp of their fundamental chemical structure. Let's explore the building blocks and organization of these essential biomolecules, with a focus on "what is the chemical composition of carbohydrates".
Unveiling the Basic Building Blocks: Monosaccharides
The simplest carbohydrate units are called monosaccharides. These are single sugar molecules, the monomers from which all other carbohydrates are built.
The Elements Involved: C, H, and O
- The core components of any monosaccharide are carbon (C), hydrogen (H), and oxygen (O).
- The general formula for a monosaccharide is (CH₂O)ₙ, where 'n' represents the number of carbon atoms. For example, glucose (a common sugar) has 6 carbon atoms, giving it the formula C₆H₁₂O₆.
- This formula highlights the ratio of hydrogen to oxygen: it's always 2:1, just like in water (H₂O). This characteristic is where the name "carbohydrate" originates - essentially "hydrated carbon."
Common Monosaccharides: Glucose, Fructose, and Galactose
These three monosaccharides are particularly important in human nutrition.
- Glucose: Often referred to as "blood sugar," glucose is the primary source of energy for our cells.
- Fructose: Found in fruits and honey, fructose is notably sweeter than glucose.
- Galactose: Rarely found on its own in nature, galactose typically combines with glucose to form lactose, the sugar in milk.
Isomers: Same Formula, Different Structure
Despite sharing the same chemical formula (C₆H₁₂O₆), glucose, fructose, and galactose have different structural arrangements. This difference in arrangement leads to distinct properties, like varying sweetness levels and how they are metabolized by the body.
From Single Units to Chains: Disaccharides and Polysaccharides
Monosaccharides can link together to form more complex carbohydrates.
Disaccharides: Two Sugars Linked
Disaccharides are formed when two monosaccharides are joined together through a glycosidic bond, releasing a water molecule (H₂O) in the process. This type of reaction is known as dehydration synthesis.
- Sucrose (Table Sugar): Glucose + Fructose
- Lactose (Milk Sugar): Glucose + Galactose
- Maltose (Malt Sugar): Glucose + Glucose
Polysaccharides: Long Chains of Sugars
Polysaccharides are complex carbohydrates composed of many monosaccharides linked together. These can be arranged in straight chains or branched structures.
Starch: Energy Storage in Plants
- Starch is a polysaccharide composed of glucose units. It serves as the primary energy storage form in plants.
- Common sources of starch include potatoes, rice, and wheat.
- Starch exists in two forms: amylose (linear chains) and amylopectin (branched chains).
Glycogen: Energy Storage in Animals
- Glycogen is the animal equivalent of starch, also composed of glucose units.
- It's stored primarily in the liver and muscles, providing a readily available source of glucose when needed.
- Glycogen is highly branched, allowing for rapid release of glucose.
Cellulose: Structural Component in Plants
- Cellulose is another polysaccharide composed of glucose units.
- It forms the structural component of plant cell walls, providing rigidity and support.
- Unlike starch and glycogen, humans cannot digest cellulose due to the specific type of glycosidic bond present. We often refer to it as dietary fiber.
Illustrative Table: Comparing Carbohydrate Types
| Carbohydrate Type | Building Block (Monomer) | Structure | Examples | Function |
|---|---|---|---|---|
| Monosaccharide | Single sugar unit | Simple | Glucose, Fructose, Galactose | Immediate energy source |
| Disaccharide | Two sugar units | Two units linked | Sucrose, Lactose, Maltose | Quick energy source |
| Polysaccharide | Many sugar units | Complex (linear or branched) | Starch, Glycogen, Cellulose | Energy storage, structural support |
Video: Carbohydrates: The Chemistry You Need to Know (Explained)
Carbohydrates: Chemistry Explained - FAQs
What exactly are carbohydrates?
Carbohydrates are organic compounds made of carbon, hydrogen, and oxygen atoms, generally with a hydrogen-oxygen atom ratio of 2:1 (like water). They are essential biomolecules providing energy to living organisms. The chemical composition of carbohydrates is fundamentally (CH₂O)n, where n is the number of carbon atoms.
What's the difference between simple and complex carbohydrates?
Simple carbohydrates (sugars) are small molecules like glucose and fructose, offering quick energy. Complex carbohydrates (starches and fibers) are larger molecules composed of many simple sugars linked together. They take longer to digest, providing sustained energy. The chemical composition of carbohydrates applies to both, it is simply how many units are linked.
What role do carbohydrates play in the body?
Carbohydrates are the body's primary energy source. They are broken down into glucose, which fuels cells, tissues, and organs. Carbohydrates also play a role in energy storage (glycogen) and building other molecules. The chemical composition of carbohydrates directly determines how easily the body can access this energy.
How do carbohydrates differ from fats and proteins in their chemical structure?
While all three contain carbon, hydrogen, and oxygen, their ratios and arrangements differ significantly. Fats have a much higher proportion of carbon and hydrogen relative to oxygen. Proteins contain nitrogen, which carbohydrates and fats lack. Thus, the chemical composition of carbohydrates is unique from lipids and proteins.
